Our Science
The SAB BIO Platform: A Novel Immunotherapy Platform For Developing Human Anti-Thymocyte Immunoglobulin
With our unique platform, SAB BIO is the only company in the world that can produce truly polyclonal human antibodies without the need for human donors.
At SAB BIO, we are always looking for partners who share our goal of addressing pressing health challenges. Interested in partnership opportunities? Contact us here.
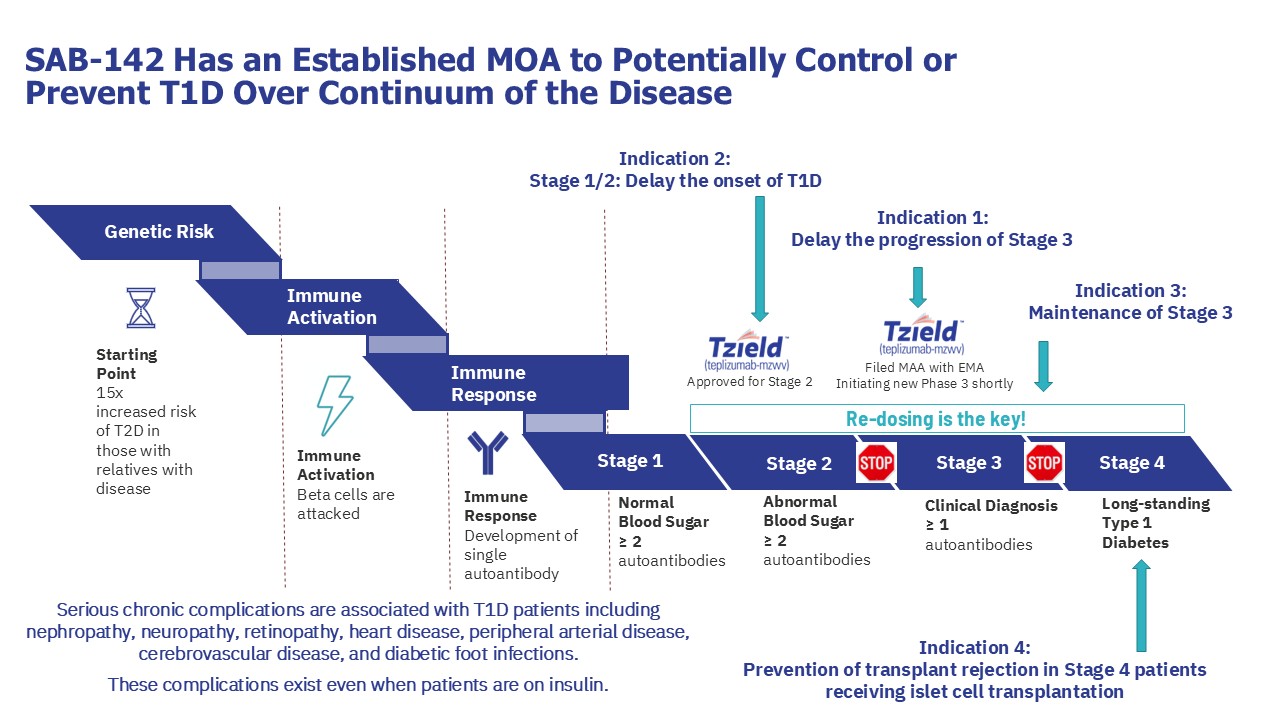
SAB-142: A Disease-Modifying Approach for Type 1 Diabetes (T1D)
Current treatment approaches for type 1 diabetes (T1D) focus primarily on daily disease management with insulin monitoring and administration to prevent complications, but these do not change the trajectory of the disease.
SAB-142 is not another insulin nor is it for chronic disease management; it is a potentially disease-modifying redosable therapy for T1D, envisioned to be administered once a year.
SAB-142: A First-In-Class, Human Alternative to Rabbit- or Equine-derived Anti-Thymocyte Globulin (ATG)
SAB-142 has a mechanism of action (MoA) that is analogous to that of FDA-approved rabbit ATG (rATG), which has been clinically validated in multiple clinical trials for T1D, demonstrating the ability to slow disease progression in patients with new or recent onset of Stage 3 T1D.
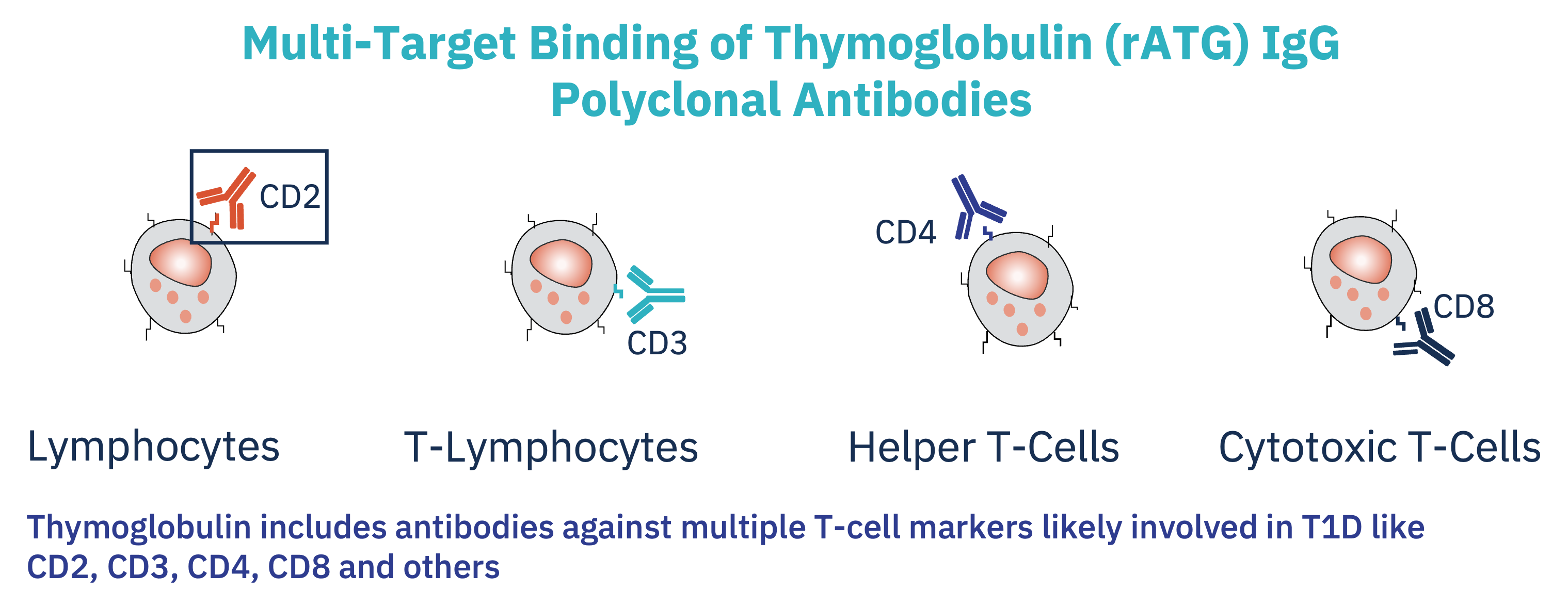
SAB-142, like rATG, directly targets multiple immune cells involved in destroying pancreatic beta cells. This includes modulation of “bad acting” T-lymphocytes like Cytotoxic T-cells, activated macrophages, and B-cells. By stopping immune cells from attacking beta cells, this treatment preserves insulin-producing beta-cells.
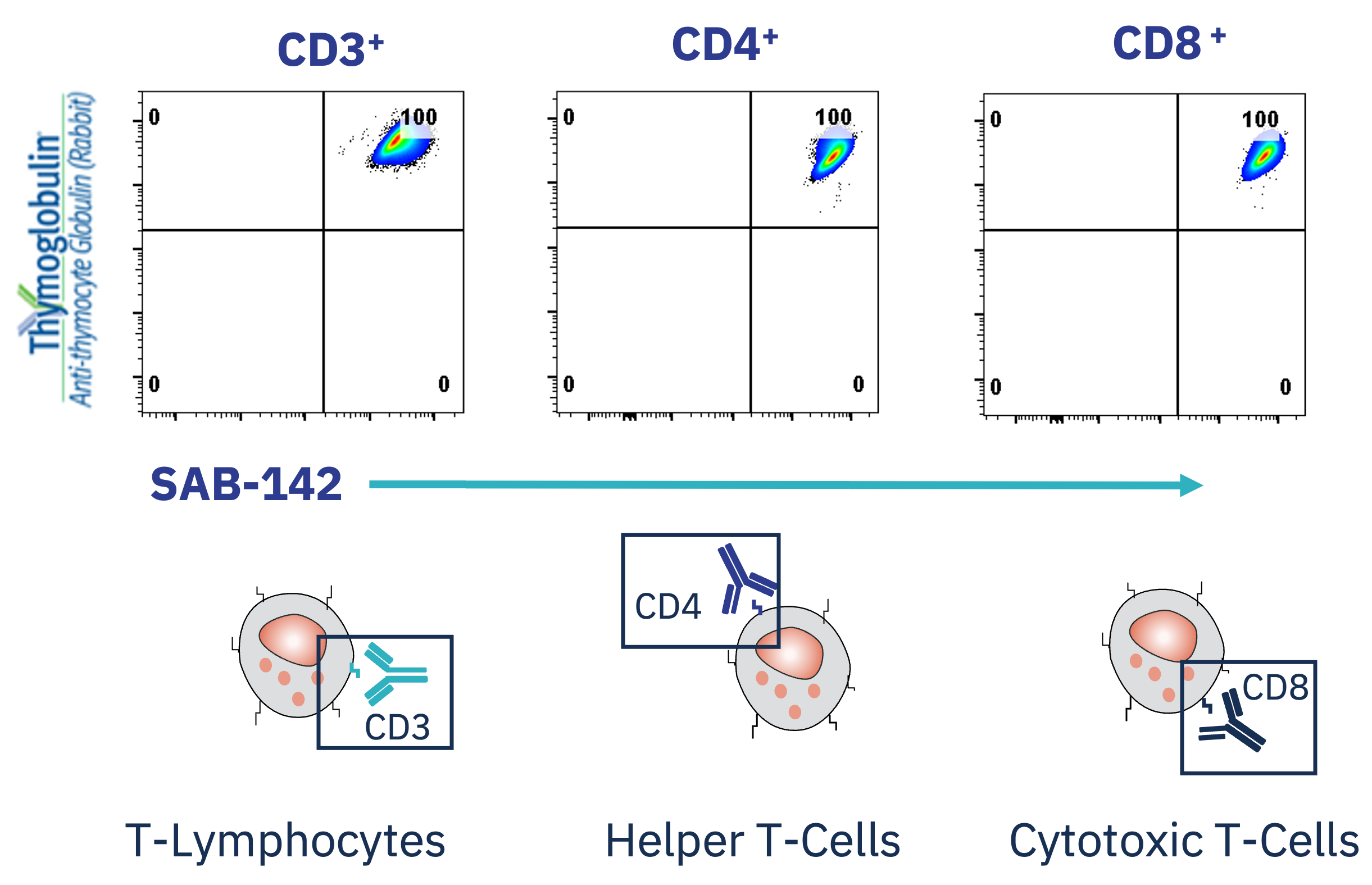
Because it is a human antibody, SAB-142 may allow for safe and consistent re-dosing for this lifelong disease without the potential risk of inducing the major immune reactions that can occur with animal immunoglobulins (IgGs).
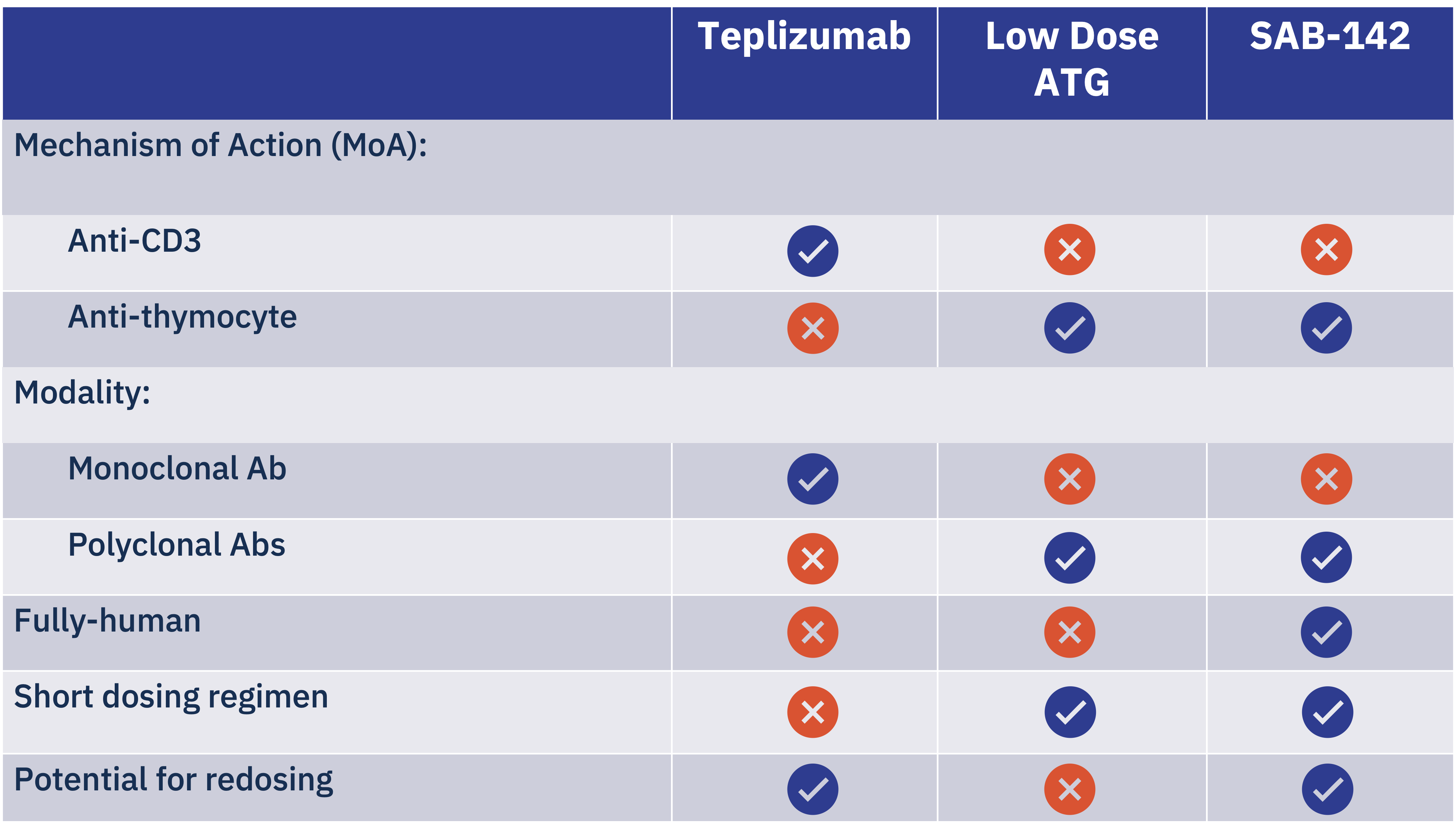
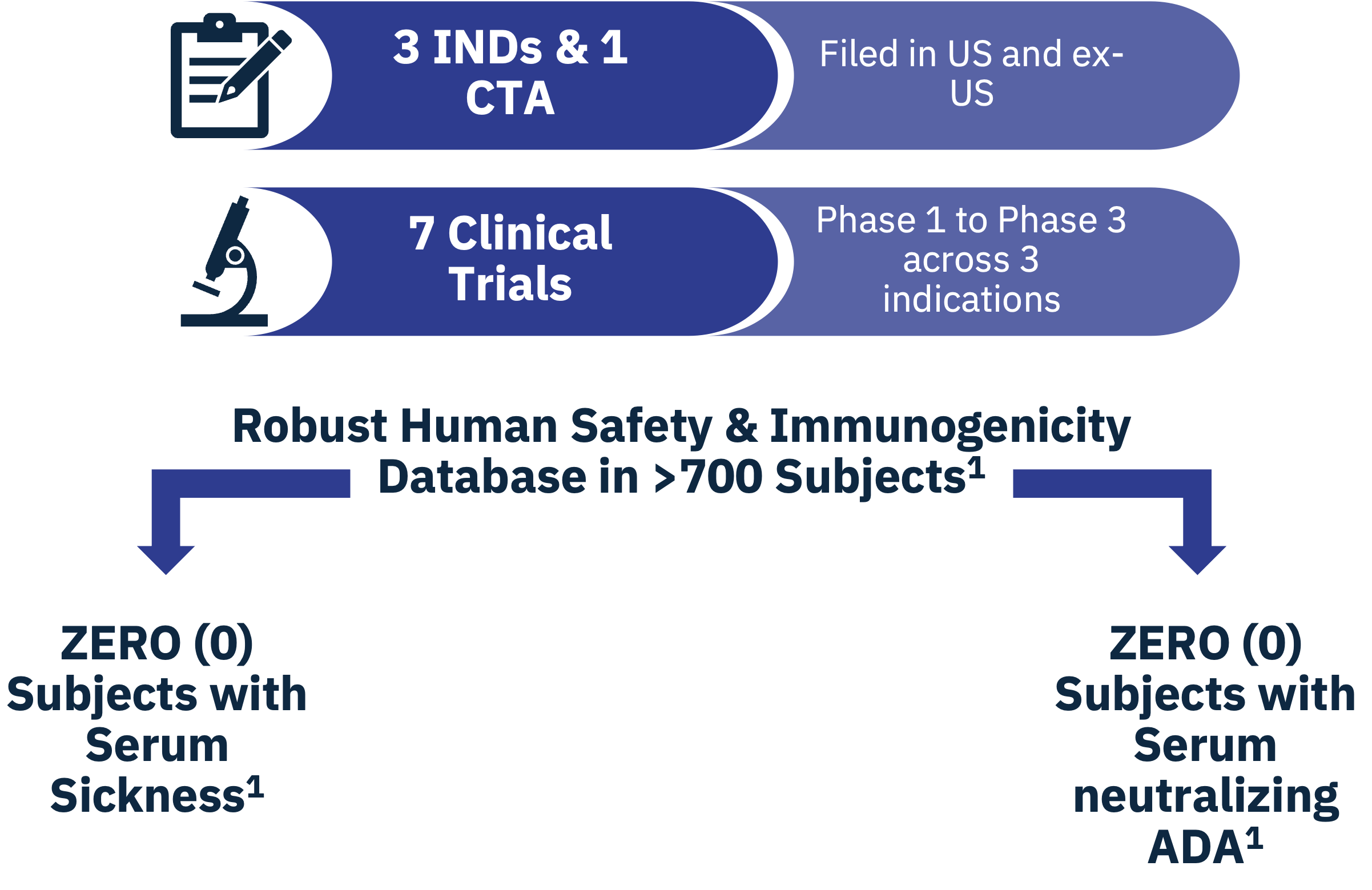
SAB-142 is anticipated to have an improved safety profile relative to rATG, which has significant safety concerns, as most patients treated with rATG develop serum sickness from exposure to non-human, rabbit IgGs. SAB BIO’s safety and immunogenicity database in more than 700 subjects with IgGs produced with SAB BIO’s platform show zero patients with serum sickness and zero patients with neutralizing anti-drug antibodies.
For more information: “Low-Dose Anti-Thymocyte Globulin Preserves C-Peptide, Reduces HbA1c, and Increases Regulatory to Conventional T-Cell Ratios in New-Onset Type 1 diabetes: Two Year Clinical Trial Data” Michael J. Haller, S. Alice Long, J. Lori Blanchfield, et al.